
“Innovate for the great majority” is the development philosophy of Qyuns. Innovation is the core driving force for our rapid development of Qyuns and has fit into all aspects of our business.
We have established an integrated R&D platform as the foundation for our continuous innovation. The platform comprises five R&D components,including (i) innovative mAb screening and function verification; (ii) antibody structure analysis; (iii) cell line screening and process development; (iv) drug formulation development;and (v) preclinical and clinical sample analysis and testing.
We have developed all of our biologic drug candidates in-house and received a number of awards recognizing our R&D capabilities. We have set up two clinical development centers in Beijing and Shanghai.

Our rabbit antibody development platform serves as the foundation of our drug discovery and development. In recent decades, there have been growing interest in the industry toward the development of rabbit mAbs as many studies have shown that the unique features of B-cell ontogeny and antibody repertoire make rabbits a valuable source for antibodies that have high affinity and specificity, which could potentially translate into strong bioactivity, and are easier to humanize, leading to lower risk of immunogenicity.
The following diagram illustrates the development workflow of rabbit mAbs.
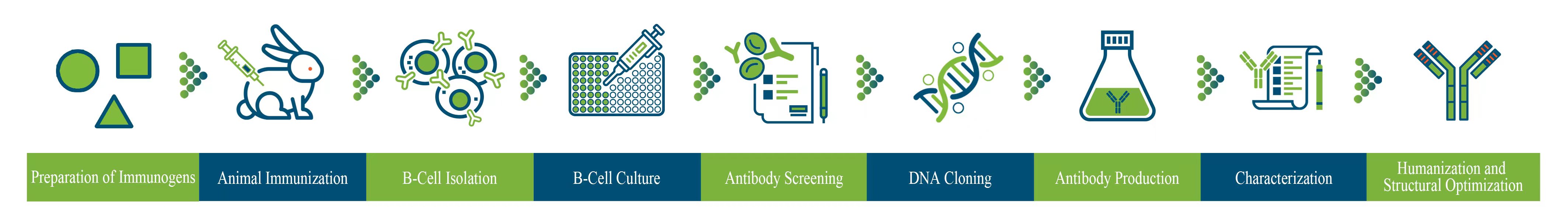
Our rabbit antibody development platform covers all core antibody R&D functions, such as antibody screening, humanization and structural optimization. We adopt an advanced B cell cloning technology that can greatly increase the access to a wider range of antigen-specific B cells and achieve high-throughput screening to isolate rare antigen-specific B cells among a high volume of such cells. Our platform not only facilitates the selection of rabbit mAbs with strong bioactivity, but also helps evaluate their viability to be further developed into commercial-grade biological drugs, aiming to avoid excessive modifications to reduce uncertainties in subsequent CMC process development.