From October 24 to 29, 2025, the American College of Rheumatology (ACR Convergence), a premier global authority conference in the rheumatology and immunology field, was held in Chicago, the United States. Qyuns Therapeutics Co., Ltd. ("Qyuns" or the "Company") presented Phase III clinical trial results in China of its self-developed CRUSEKITUG (QX002N) for the treatment of ankylosing spondylitis (AS) in an oral presentation at the ACR annual meeting.

Crusekitug (QX002N) Presented at the 2025 ACR Annual Meeting
This study, led by Professor Zeng Xiaofeng of the Rheumatology and Immunology Department at Peking Union Medical College Hospital, Chinese Academy of Medical Sciences, was a multicenter, randomised, double-blind, placebo-controlled Phase III clinical study. The study included a 48-week treatment period (comprising a 16-week double-blind treatment phase and a 32-week open-label treatment phase) and a 4-week safety follow-up period, involving 641 patients randomised in a 1:1 ratio across 58 research centers in China to receive either 160 mg of Crusekitug or a placebo (subcutaneous administration once every four weeks, Q4W). The primary endpoint of the study was the proportion of subjects achieving an ASAS40 response[1] at week 16.
CRUSEKITUG (QX002N) DEMONSTRATES OUTSTANDING EFFICACY IN PHASE III TRAIL FOR ANKYLOSING SPONDYLITIS
Study results[2] indicated that CRUSEKITUG demonstrated significant and sustained improvements in disease activity, signs and symptoms among patients with active ankylosing spondylitis (AS) who had an inadequate response to or contraindication for nonsteroidal anti-inflammatory drugs (NSAIDs), with favourable safety and tolerability profiles observed over the 16-week treatment period.
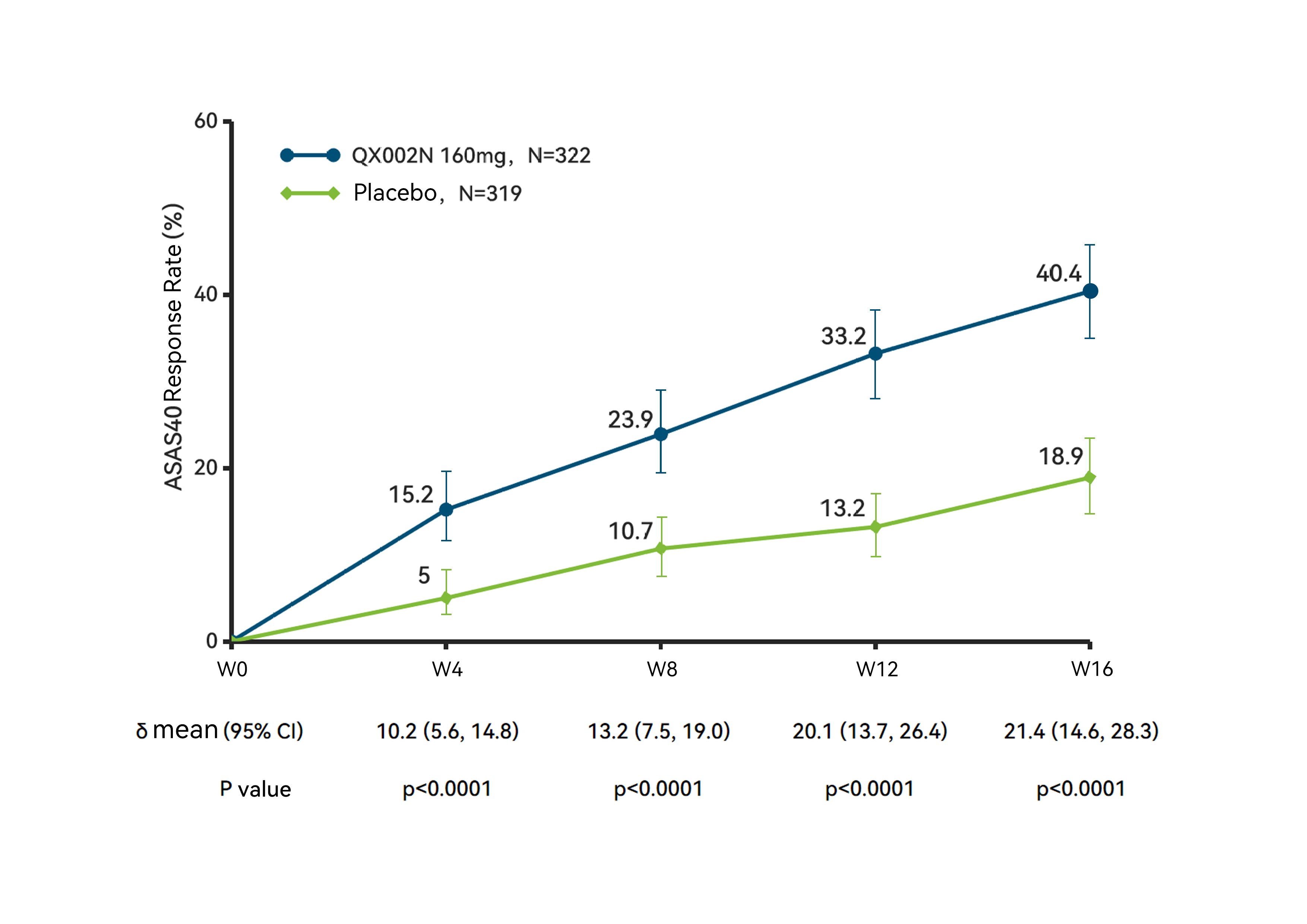
ASAS40 Response Rate, Over 16-Week Treatment Period
The results showed that at week 16, the study met its primary endpoint, with the ASAS40 response rate in the Crusekitug group was 40.4%, significantly higher than the 18.9% in the placebo group (P < 0.0001); meanwhile, the ASAS20 response2 [3]rate in the Crusekitug group was 65.2%, also significantly higher than that in the placebo group (P < 0.0001), indicating that Crusekitug effectively alleviates the symptoms and signs of AS patients across multiple dimensions, including pain and spinal function.
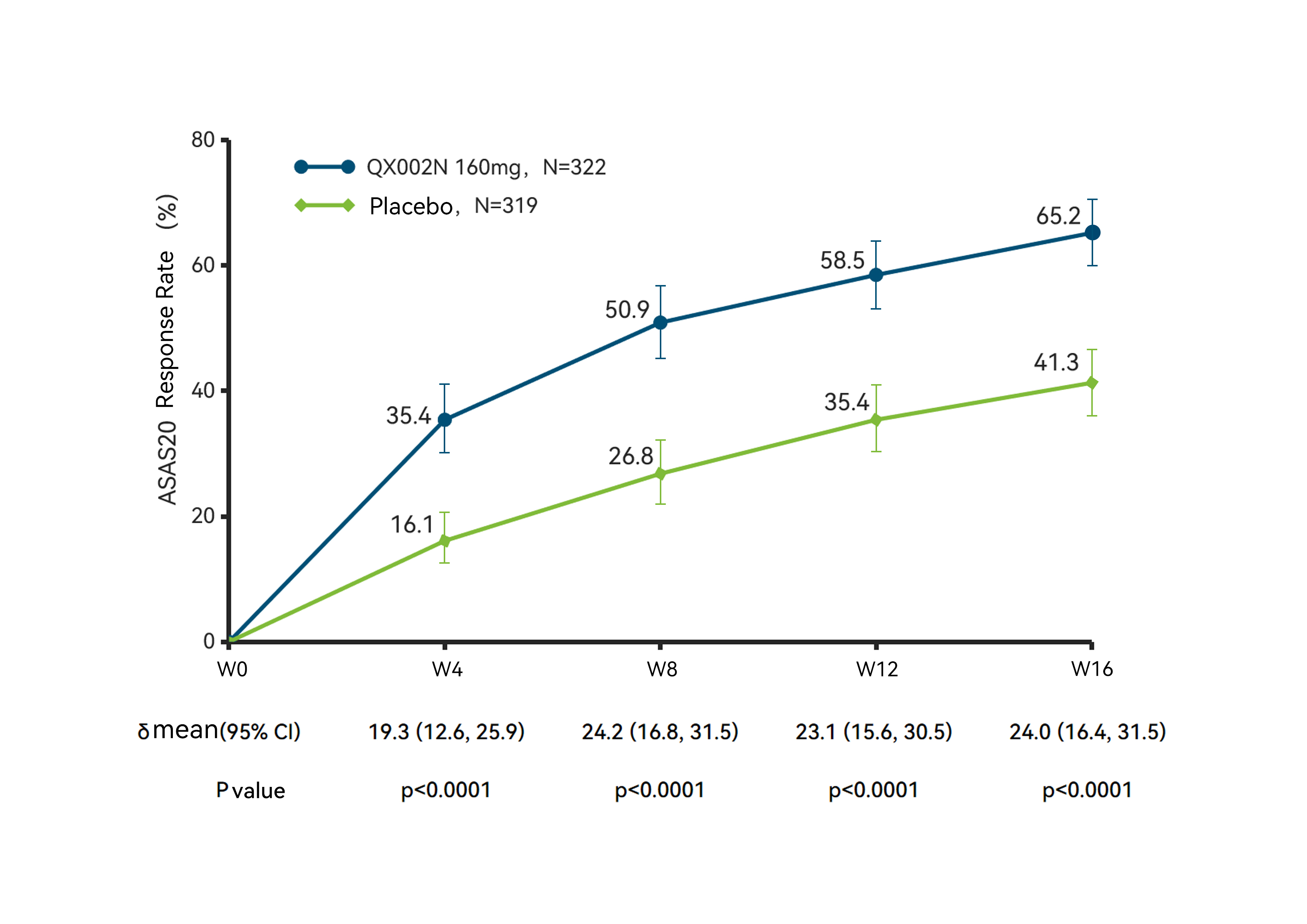
ASAS20 Response Rate, Over 16-Week Treatment Period
Secondary endpoint results demonstrated that Crusekitug also exhibited significant superiority across multiple disease assessment measures, including disease activity, physical function, spinal mobility and life quality.
IMAGING DATA CONFIRMED A SIGNIFICANT RESOLUTION OF INFLAMMATORY EDEMA
In addition to the improvements in clinical symptoms and spinal function, the study also assessed inflammation in patients’ spine and sacroiliac joints via magnetic resonance imaging (MRI). Intra-articular or intraosseous inflammation, bone destruction and new bone formation are recognized as key pathophysiological processes of axial spondyloarthritis (axSpA). MRI-detectable subchondral bone marrow edema is present in biopsy specimens in the early stages of the disease course, reflecting active inflammation[4,5]. The Spondyloarthritis Research Consortium of Canada (SPARCC) score, as an MRI-specific metric, visually quantifies edema in the spine and sacroiliac joints, thereby objectively reflecting disease activity.
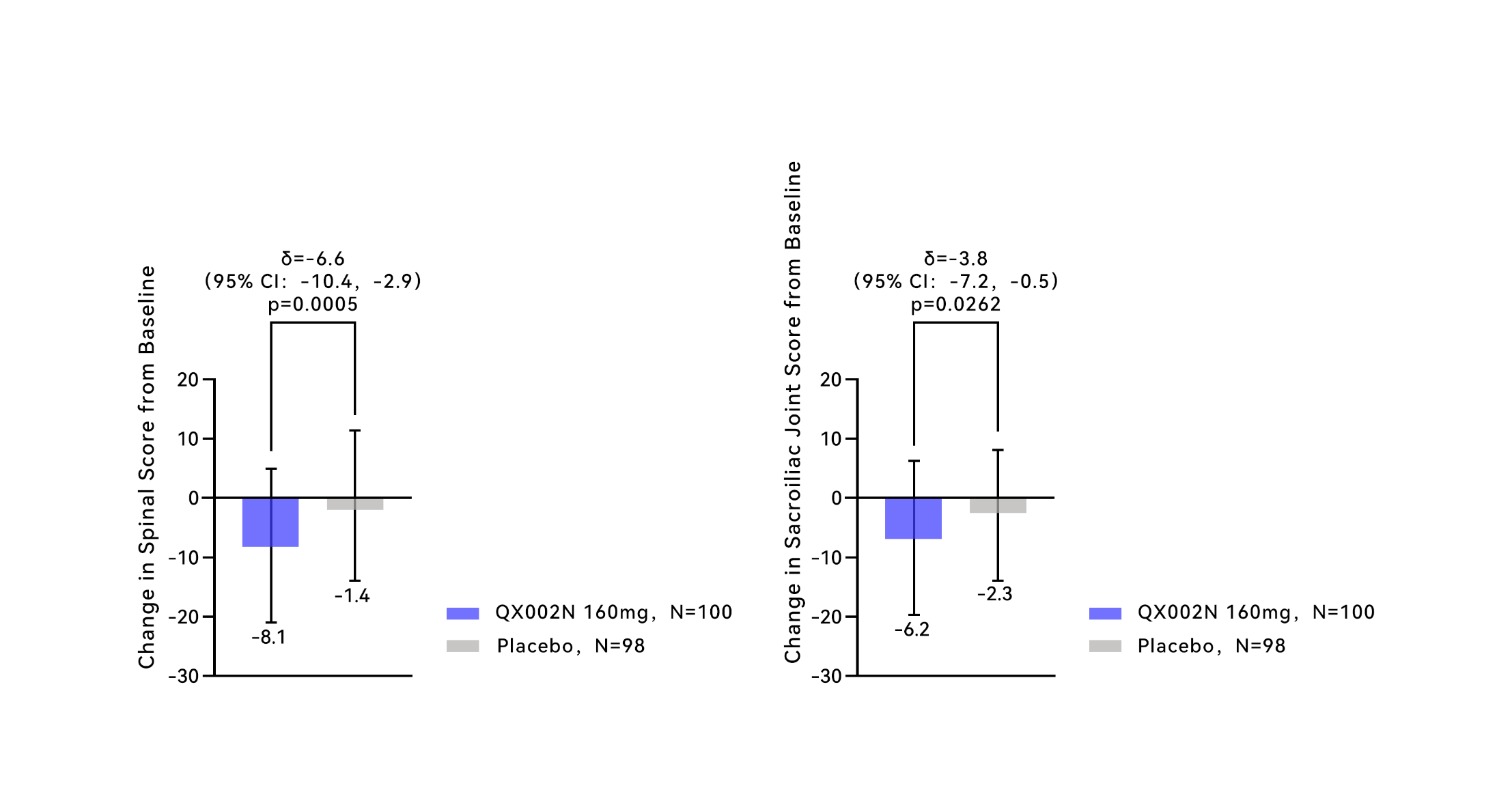
ASAS20 Response Rate, Over 16-Week Treatment Period
The treatment results at week 16 showed that the change from baseline in the spinal score was -8.1 for the Crusekitug group and the change from baseline in the sacroiliac joint score was -6.2, both significantly better than the -1.4 and -2.3 observed in the placebo group, respectively. This indicates that Crusekitug effectively reduces edema and inflammation in the spine and sacroiliac joints of subjects, providing objective imaging evidence for the drug’s ability to suppress disease activity.
In terms of safety, at week 16, the incidence of treatment-emergent adverse events (TEAEs) and serious adverse events (SAEs) in the Crusekitug group was similar to that in the placebo group, and most TEAEs were mild to moderate, indicating overall favourable safety profile.
The excellent clinical symptom relief efficacy and clear imaging evidence position Crusekitug as a potential new treatment option for AS patients. The Company will also accelerate the registration and submission process of such drug, striving for its early approval and market launch.
ABOUT CRUSEKITUG (QX002N)
Crusekitug is a high-affinity monoclonal antibody targeting IL-17A. IL-17A is a member of the IL-17 superfamily of cytokines and a key player in the pathological mechanism of various autoimmune diseases. IL-17A enhances chronic inflammation by inducing the release of and working in synergy with pro-inflammatory cytokines such as interleukin-6 (IL-6) and chemokine CXCL1. Additionally, IL-17A is involved in the regulatory mechanism of bone remodeling and has been identified as a major factor in AS pathogenesis. Crusekitug is designed to specifically bind to IL-17A, including IL-17AA and IL-17AF, thereby blocking their binding to the intended receptor complex, comprised of IL-17RA and IL-17RC, and preventing the subsequent activation of several pro-inflammatory signaling pathways, thereby inhibiting the onset and progression of inflammation.
ABOUT ANKYLOSING SPONDYLITIS
Ankylosing spondylitis (AS) is a chronic progressive inflammatory disease characterized primarily by spinal joint inflammation, which leads to reduced joint mobility and spinal stiffness over time. According to data from Frost & Sullivan, there were 3.9 million AS patients in China in 2021, and the number is projected to reach 4.0 million by 2030. To date, the only biological drugs approved for the clinical treatment of AS in China are TNF inhibitors and IL-17 inhibitors, both of which are recommended therapies for AS patients with persistently active disease following treatment with NSAIDs. Among these two classes of biological therapies, IL-17A inhibitors provide significant clinical benefits to patients who are TNF-α inhibitor-naïve, as well as those who are intolerant to TNF-α inhibitors or unable to achieve adequate disease control.
DISCLAIMER
*This news release is intended to share the latest progress updates on the R&D efforts of the Company (or its partners) and is not for advertising purposes. It does not constitute a recommendation for any drugs and/or indications. For any related diseases or medication needs, please consult a qualified healthcare professional.
*This news release may contain certain forward-looking statements, which are inherently subject to significant risks and uncertainties. When words such as "anticipate," "believe," "predict," "expect," "plan," "intend" and other similar expressions are used, insofar as they relate to the Company, they shall be deemed to be forward-looking statements. The Company has no obligation to continuously update these predictive statements. These forward-looking statements are based on the current views, assumptions, expectations, estimates, projections and understandings of the Company’s management regarding future events at the time of making such statements. They do not constitute guarantees of future developments nor reliable indicators of future performance. We hereby explicitly caution you that you should not rely on any forward-looking statements. Forward-looking statements are subject to various risks, uncertainties and other factors (including but not limited to general market conditions, regulatory changes, geopolitical tensions, or data limitations and changes), some of which are beyond the Company’s control and difficult to predict. Therefore, due to future changes and developments in our business, competitive environment, political, economic, legal and social conditions, actual results may differ materially from the information contained in the forward-looking statements. The Company, its directors and employee agents shall not be liable for any obligation to update, revise or supplement such forward-looking statements, nor for any liability arising from the failure or inaccuracy of any forward-looking statements.
NOTES AND REFERENCES
[1] At least three out of the four key domains in the Assessment of SpondyloArthritis International Society (ASAS) response criteria achieved a 40% improvement with an absolute increase of ≥2 points, and no worsening was observed in the remaining domain(s).
[2] Zeng X, Zhang S, LIU S, Li F, wang x, SUN L, Du H, Shi G, li y, zhang h, Zhang L, Wu J, zhou m, gu z, zhao y, fang m, Song Q, wang t. Effect of QX002N on Clinical and Radiographic Outcomes in Ankylosing Spondylitis: Results from a Phase III Randomized, Double-blind, Placebo-Controlled Study [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/effect-of-qx002n-on-clinical-and-radiographic-outcomes-in-ankylosing-spondylitis-results-from-a-phase-iii-randomized-double-blind-placebo-controlled-study/. Accessed October 21, 2025.
[3] Under the Assessment of Spondyloarthritis international Society (ASAS) response criteria, at least 3 out of the four key indicators must achieve a 20% improvement with an absolute improvement of ≥1 point, and the remaining indicator(s) must not deteriorate.
[4] Navarro-Compán V, Sepriano A, Capelusnik D, Baraliakos X. Axial spondyloarthritis. The Lancet. 2025;405(10473):159-172.
[5] Maksymowych WP, Inman RD, Salonen D, et al. Spondyloarthritis research Consortium of Canada magnetic resonance imaging index for assessment of sacroiliac joint inflammation in ankylosing spondylitis. Arthritis & Rheumatism. 2005;53(5):703-709.

 hit:
次
hit:
次